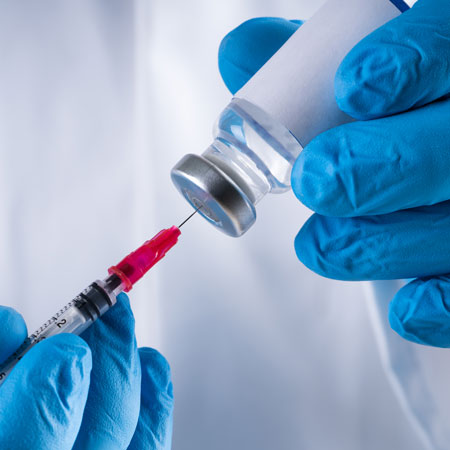
By injecting the monkeypox vaccine into the skin rather than under the skin, a smaller dose is sufficient to provide a protective effect. So the public health authority is expanding Its recommendations for whom the vaccine can be given also include people who have a “significantly high risk” of infection.
In the past, the vaccine was recommended for people who were part of an infection tracking system and who had close physical contact with someone who had monkeypox.
It is with great pleasure that we are now also recommending that the scope of use be expanded and that more people receive preventive treatment. Although we still have a shortage of a monkeypox vaccine, the vaccination can now be offered to more people, Public Health Agency Director-General Karen Tegmark Wessel says, in a press release.
The vaccine used is manufactured by the North Bavarian Danish company and called Imvanex in the European Union and Jynneo in the United States. The European Union preparedness body Hera has allocated vaccines to Sweden, which, using previous vaccination technology, were considered sufficient for about 1,500 people.
With the new injection method, vaccine doses can be reduced from 0.5ml to 0.1ml per dose, according to European Medicines Agency EMA. That is, the vaccine is enough for five times the number, that is, 7,500 people.
Doctors in clinics where the vaccine is available decide who should be offered the vaccine.
Until now 154 cases of monkeypox It has been confirmed in Sweden and cases have been reported mainly from the capital districts of Stockholm, Skene and Västra Götaland.
Lakartidningen.se

“Extreme tv maven. Beer fanatic. Friendly bacon fan. Communicator. Wannabe travel expert.”








More Stories
Why Rare Earth Metals for Electric Cars Are Crucial for Modern Mobility
“We want to promote critical rules approach”
“A lot happened during the trip,” Jönköping County Council